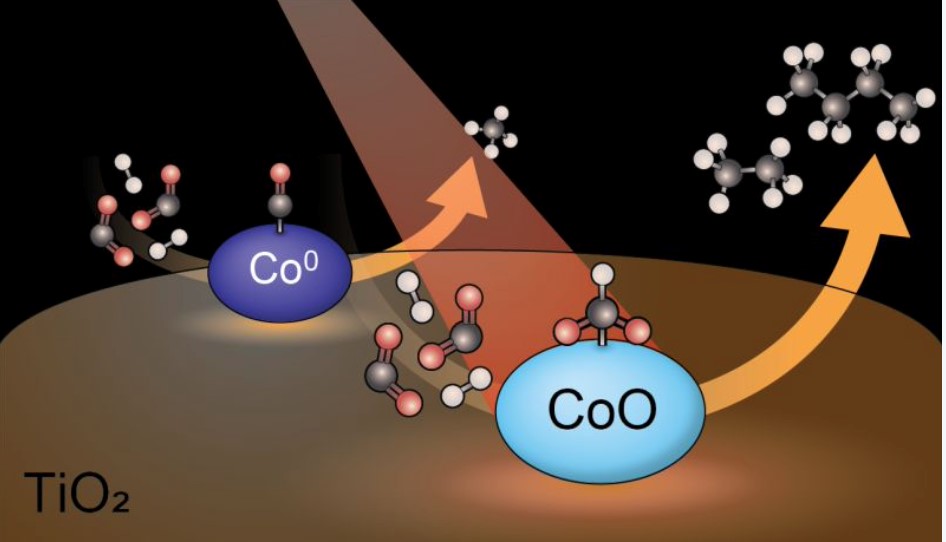
ICC scientists, in cooperation with the Paul Scherrer Institute in Switzerland, have discovered new fundamental insights into the reaction mechanism of CO2 hydrogenation. CO2 hydrogenation is one of a set of important reactions that can help solve the problems related to global warming caused by CO2 in the atmosphere. The reaction converts the harmful CO2 molecule back to useful fuels, using – in principle cheap – green hydrogen. Up to now, most scientists assumed that cobalt metal particles were the most active phase in the hydrogenation, but PhD student Iris ten Have found that on a titania (TiO2) support, it is actually an oxide of cobalt (CoO), not the metal phase (Co0), that is responsible for the most efficient catalysis. The work is reported in Nature Communications.
The team used cobalt-based catalysts made from a non-reducible support (silica and alumina) and a reducible support (ceria and titania) in combination with advanced spectroscopic tools, such as Modulated Excitation (ME) Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS), to demonstrate how the interaction with the support material can tune the oxidation state of the cobalt, and thereby unlock new reaction pathways. An interesting consequence is that the catalyst becomes more active in carbon coupling reactions, and significant amounts of larger hydrocarbon molecules with two or more carbon atoms, like ethane, ethylene and propylene are observed. This expands the toolbox of chemists to convert harmful CO2 to larger useful molecules.
Press release UU: https://www.uu.nl/en/news/what-if-we-could-turn-co2-into-something-useful
Publication:
Uncovering the reaction mechanism behind CoO as active phase for CO2 hydrogenation, Iris ten Have, Joyce Kromwijk, Matteo Monai, Davide Ferri, Ellen Sterk, Florian Meirer and Bert Weckhuysen, Nature Communications volume 13, Article number: 324 (2022), DOI: https://doi.org/10.1038/s41467-022-27981-x